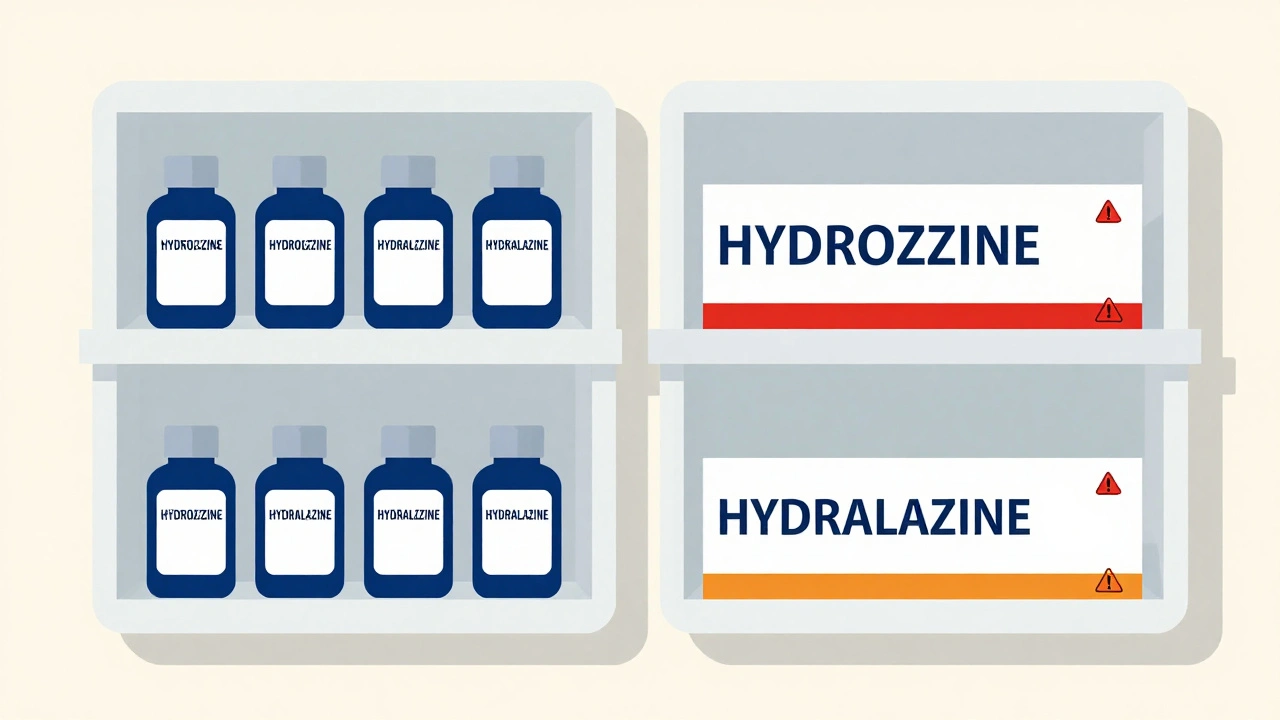
OTC Sunscreens: How SPF, Broad Spectrum, and Reapplication Actually Work
OTC sunscreens aren't just for the beach. Learn how SPF, broad spectrum, and reapplication really work to protect your skin from sun damage and skin cancer - backed by science and real-world testing.