Active Ingredients in OTC Drugs Explained for Shoppers
 Dec, 2 2025
Dec, 2 2025
Every time you grab a bottle of pain relief, cold medicine, or allergy pill off the shelf, you’re making a decision based on packaging, price, or brand. But what’s actually inside that pill or liquid? That’s the active ingredient-the part that does the work. And if you don’t know what it is, you could be risking your health without even realizing it.
What Exactly Is an Active Ingredient?
The active ingredient is the chemical in an over-the-counter (OTC) drug that causes the intended effect. It’s not the flavor, color, or filler. It’s the thing that reduces your fever, stops your cough, or eases your headache. For example, if you take Tylenol, the active ingredient is acetaminophen. If you take Advil, it’s ibuprofen. Same medicine, different brand names. The U.S. Food and Drug Administration (FDA) requires every OTC product to list active ingredients clearly on a standardized label called the Drug Facts label. This label isn’t optional-it’s the law. And it’s the only reliable way to know what you’re really taking.How to Read the Drug Facts Label
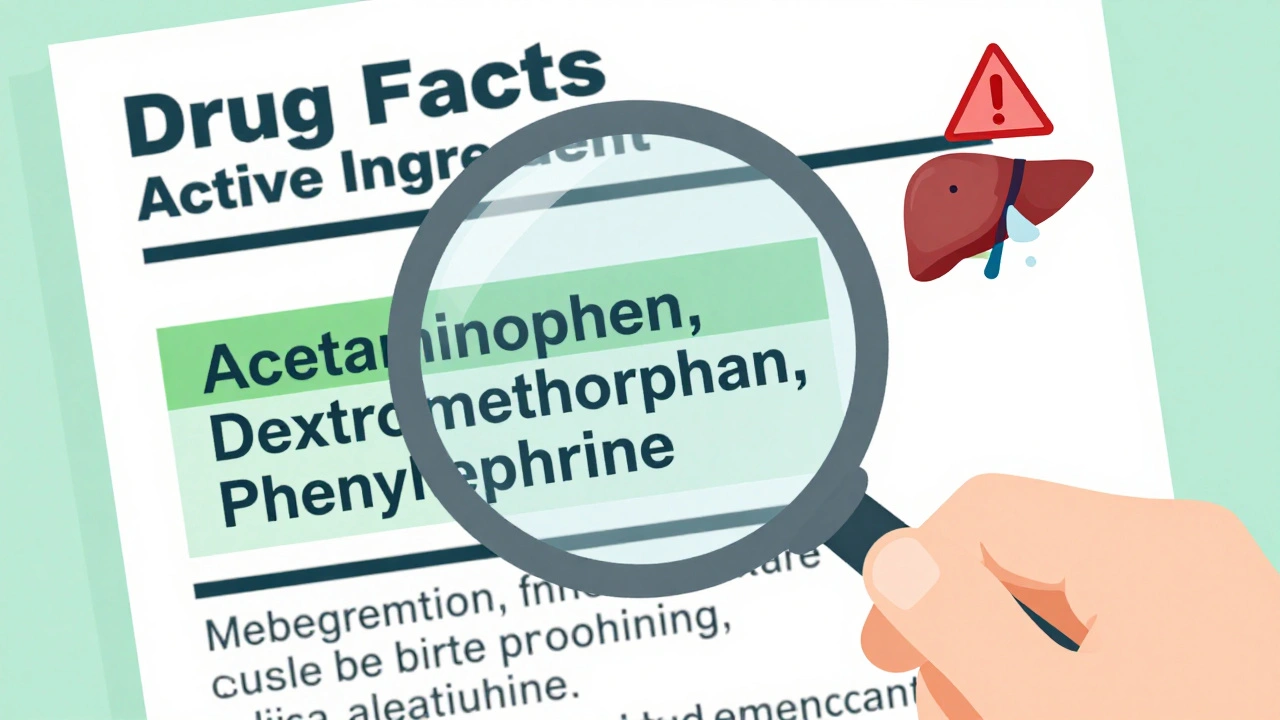
The Drug Facts label has seven sections, but the most important one is always first: Active Ingredient(s). Here’s how to use it:- Find the Active Ingredient section. It’s always at the top. No exceptions.
- Look for the name and amount. It will say something like “acetaminophen 325 mg” or “ibuprofen 200 mg.” That’s the exact dose per tablet, capsule, or teaspoon.
- Check for multiple ingredients. Cold and flu products often combine several active ingredients. A product might list: “acetaminophen 325 mg, dextromethorphan 10 mg, phenylephrine 5 mg.” That’s three different drugs in one pill.
- Compare to other meds you’re taking. If you’re already taking a prescription painkiller or another OTC product, check if it contains the same active ingredient. Double-dosing is dangerous.
Spending just 45 seconds reading this section can cut your risk of accidental overdose by nearly 70%, according to Nationwide Children’s Hospital. Most people skip it-until something goes wrong.
Common Active Ingredients and What They Do
You don’t need to memorize every chemical name, but knowing these top 5 will help you avoid mistakes:- Acetaminophen (Tylenol, TheraFlu, Percocet®): Reduces fever and pain. Found in more than 600 OTC products. Overdose can cause liver failure. Max daily dose: 4,000 mg for adults.
- Ibuprofen (Advil, Motrin, Nuprin): Reduces pain, fever, and inflammation. Used for headaches, menstrual cramps, arthritis. Max daily dose: 1,200 mg OTC. Higher doses require a prescription.
- Diphenhydramine (Benadryl, Tylenol Nighttime): An antihistamine that causes drowsiness. Used for allergies and sleep. Found in many nighttime cold medicines. Can cause confusion in older adults.
- Dextromethorphan (Robitussin, DayQuil): A cough suppressant. Often abused when taken in large doses. Can cause hallucinations and heart problems if misused.
- Phenylephrine (Sudafed PE, Claritin-D): A decongestant. Raises blood pressure. Avoid if you have heart disease or high blood pressure.
Here’s the catch: One product might have acetaminophen, and another might have ibuprofen. But if you take both, you’re not getting “double the relief.” You’re just doubling your risk of side effects.
Why Brand Names Are Misleading
You’ve probably seen ads for “new improved” cold medicines with flashy packaging. But here’s the truth: Brand names don’t tell you what’s inside.Take this example: Tylenol, Excedrin, TheraFlu, and NyQuil all contain acetaminophen. So does store-brand generic cold medicine. If you take two of these in one day, you could easily hit the 4,000 mg daily limit-and that’s when liver damage starts.
A 2023 Consumer Reports survey found that only 28% of people could correctly identify that Aleve contains naproxen sodium. Most assumed it was something else. That’s not just ignorance-it’s a public health problem.
The FDA says 70% of OTC medication errors happen because people don’t check active ingredients. They assume “it’s just a cold medicine,” or “this one doesn’t have painkiller in it.” But it does. And they didn’t check.
The Hidden Danger: Combining Medications
The biggest risk isn’t taking one wrong pill. It’s taking two or more that have the same active ingredient.Here’s a real story: A man took Tylenol for a headache. Later, he took TheraFlu Nighttime for his cold. He didn’t realize both had acetaminophen. He took three doses. By morning, his liver enzymes were skyrocketing. He ended up in the ER. He survived-but barely.
This happens every day. In 2022, Reddit’s r/Pharmacy thread had over 280 comments from people who accidentally overdosed on acetaminophen. One user wrote: “I took two Tylenol and one TheraFlu. I thought TheraFlu was just for congestion. I didn’t even know it had painkiller in it.”
Same goes for dextromethorphan. People take cough syrup and then take a cold tablet with the same ingredient. The result? Dizziness, nausea, rapid heartbeat-even seizures.
Doctors and pharmacists recommend a simple trick: Write down every active ingredient on a piece of paper before you buy anything. If two products have the same one, don’t take both.
What’s New in OTC Drug Safety (2025)
The rules are changing. In 2020, Congress passed the CARES Act, which forced the FDA to turn its OTC drug review system from a slow administrative process into a binding legal framework. By December 2023, every single OTC monograph had to be finalized-or the product was pulled from shelves.That means:
- Acetaminophen tablets can no longer be sold in 1,000 mg doses. The max is now 650 mg per tablet in prescription combinations. OTC products are still capped at 500 mg per tablet, but the FDA is pushing for lower limits.
- All new OTC products must include a “Liver Warning” box if they contain acetaminophen.
- By 2026, all OTC drugs sold in the U.S. will need a QR code on the package that links to a digital Drug Facts label with full ingredient details-including allergens in inactive ingredients.
Why? Because people are still getting hurt. A 2023 study showed that 162 people died between 2012 and 2022 from overdosing on loperamide-an OTC anti-diarrhea drug that acts like an opioid. Consumers didn’t know it was an opioid. They thought it was “just a stomach pill.”
What You Can Do Today
You don’t need a pharmacy degree to stay safe. Just follow these four steps every time you buy an OTC drug:- Look for the Drug Facts label. If it’s not there, don’t buy it.
- Read the Active Ingredient section first. Don’t skip to Uses or Warnings.
- Write down the name and amount. Use your phone notes or a small notepad.
- Compare to anything else you’re taking. Even vitamins or supplements can have hidden active ingredients.
The FDA offers a free printable Active Ingredient Reference Chart with 35 common ingredients and their max daily doses. It’s available on their website. Print one. Keep it in your wallet or taped to your medicine cabinet.
And if you’re buying for a child, double-check the dose. Children’s Motrin has ibuprofen. Children’s Zyrtec has cetirizine. They’re not interchangeable. One treats pain. The other treats allergies. Mixing them up can be dangerous.
Final Thought: Your Medicine Cabinet Isn’t a Lottery
OTC drugs are safe-when used correctly. But they’re not harmless. They’re powerful chemicals. And if you treat them like candy, they’ll treat you like a statistic.You wouldn’t take two different painkillers without knowing what’s in them. So why do it with OTCs? The answer is simple: You didn’t know you needed to check.
Now you do.
How do I know if two OTC medicines have the same active ingredient?
Always check the Drug Facts label under the "Active Ingredient" section. Even if the brand names are different-like Tylenol and CVS Health Pain Relief-they may both contain acetaminophen. Write down the exact name and amount (e.g., "acetaminophen 500 mg"). If two products have the same ingredient, don’t take them together.
Is it safe to take OTC medicine with a prescription drug?
Not always. Many OTC drugs interact with prescriptions. For example, ibuprofen can increase bleeding risk if you’re on blood thinners. Acetaminophen can overload your liver if you’re taking opioids like oxycodone. Always check with your pharmacist before mixing any OTC medicine with a prescription.
Why do some OTC drugs have lower doses than prescription versions?
OTC drugs are meant for short-term, mild symptoms. Higher doses require medical supervision because they carry greater risks. For example, OTC ibuprofen is capped at 200 mg per tablet, while prescription versions go up to 800 mg. The lower dose reduces the chance of stomach bleeding or kidney damage in people who don’t need strong treatment.
Can I trust store-brand OTC drugs?
Yes, as long as the active ingredient matches the brand-name version. Store brands are required by law to contain the same active ingredient, in the same amount, and with the same labeling as name-brand products. The only differences are in inactive ingredients (like dyes or fillers), which rarely affect safety. Save money-just check the Drug Facts label.
What should I do if I think I took too much of an OTC drug?
Call Poison Control immediately at 1-800-222-1222 in the U.S. or your local emergency number. Don’t wait for symptoms. Acetaminophen overdose, for example, can damage your liver before you feel anything. Keep the medicine bottle handy when you call-they’ll need the exact active ingredient and amount.
Next time you reach for an OTC medicine, pause. Read the label. Know what you’re taking. It’s not just smart-it could save your life.
Mindy Bilotta
December 3, 2025 AT 14:18Just read the Drug Facts label like my pharmacist told me last year. Changed my life. No more mixing Tylenol with cold meds. Seriously, 45 seconds could save your liver.
Michael Bene
December 4, 2025 AT 13:14Oh wow, so you mean that fancy $12 bottle of ‘UltraPower Cold Relief’ is just acetaminophen + dextromethorphan + phenylephrine in a glittery box? And the $4 generic is literally the same thing? My entire life has been a lie. I’ve been paying for marketing, not medicine. The FDA should charge companies for emotional manipulation. Also, why does dextromethorphan taste like burnt plastic? I’m not okay.
Jim Schultz
December 6, 2025 AT 05:36Look. I’ve worked in ER for 18 years. I’ve seen people come in with liver failure because they thought ‘Nighttime’ meant ‘just sleep aid’ and didn’t check the label. This isn’t theory. This is blood on the floor. You don’t need a degree to read a label. You need to stop being lazy. And if you’re still buying ‘new and improved’ stuff? You’re the reason this post exists.
Katherine Gianelli
December 7, 2025 AT 11:53I used to just grab whatever looked cheapest or had the best sale sticker. Then my grandma had a bad reaction because she took two different ‘sleep aids’-both had diphenhydramine. She didn’t know. Neither did I. We’re not dumb. We’re just not taught this stuff. Thank you for making it so clear. I printed your chart. Taped it to my fridge next to the milk.
Joykrishna Banerjee
December 8, 2025 AT 17:10Of course the FDA is ‘updating’ labels. But let’s be real-this is just corporate control disguised as safety. They’re forcing QR codes because they want to track your medicine consumption. Next thing you know, they’ll be syncing your OTC intake with your Fitbit. Wake up. The system wants you dependent on their ‘verified’ labels. The real danger? Trusting them.
Archie singh
December 9, 2025 AT 11:20People still don’t know acetaminophen is in 600+ products? This isn’t ignorance-it’s a cultural failure. You can’t outsource your health to packaging. If you can’t read a label, maybe you shouldn’t be allowed to buy pills. This isn’t rocket science. It’s elementary school. And yet here we are.
Ignacio Pacheco
December 10, 2025 AT 18:04So let me get this straight-you’re telling me I’ve been overpaying for ‘Advil’ when ‘Ibuprofen’ from the dollar store does the same thing? And I’ve been doing this for 12 years? Wow. I feel like I’ve been scammed by a cereal box. Also, why does every cold medicine taste like regret?
Francine Phillips
December 12, 2025 AT 10:03My mom takes 3 different OTC meds every night. I’m gonna print this and hand it to her with a highlighter. She’ll probably ignore it but at least I tried.
Ethan McIvor
December 13, 2025 AT 19:28It’s funny how we trust our phones more than our medicine cabinets. We’ll check reviews for a toaster but grab a bottle of pills without a second thought. Maybe we need a ‘Drug Facts’ app that scans the label and says ‘HEY YOU’RE DOUBLING UP ON ACETAMINOPHEN’ in a loud voice. Just a thought.
parth pandya
December 15, 2025 AT 08:40Bro I just checked my cold medicine-its acetaminophen 500mg and I took 2 last night. Oh shit. I think I just risked my liver. I’m calling poison control now. Thanks for the wake up call.
Albert Essel
December 15, 2025 AT 21:21One of the most important public health messages I’ve seen in years. Simple. Clear. Necessary. I’ve shared this with my entire family. My teenage daughter now checks labels before she takes anything. That’s progress.
Kidar Saleh
December 16, 2025 AT 05:44Back in the UK, we used to have ‘pharmacist-only’ sections for these exact reasons. No one just grabs a bottle off the shelf. We’re lucky here in the States that we even have the Drug Facts label. It’s not perfect, but it’s a start. Still, I’m shocked so many people don’t know how to read it. Maybe we need it taught in high school biology.
Myson Jones
December 16, 2025 AT 20:08As a healthcare educator, I commend this post. The disconnect between consumer behavior and pharmacological literacy is alarming. I recommend integrating this content into community health workshops. The QR code initiative, while technologically progressive, must be accompanied by accessible educational outreach. Let us not mistake innovation for understanding.
Brian Perry
December 17, 2025 AT 23:32Okay so I just took NyQuil for my cold and then realized I already took Tylenol this morning. I think I’m gonna die. I’m typing this from my bathroom floor. My vision is blurry. Is this normal? Someone tell me if I’m gonna wake up with a new liver or if I’m just gonna become a Reddit cautionary tale. Also, why does everything taste like metal now?
Mindy Bilotta
December 19, 2025 AT 14:51Call Poison Control. NOW. 1-800-222-1222. Don’t wait. Don’t Google it. Just call. You’re not alone. I’ve been there. You’re gonna be okay if you act fast.