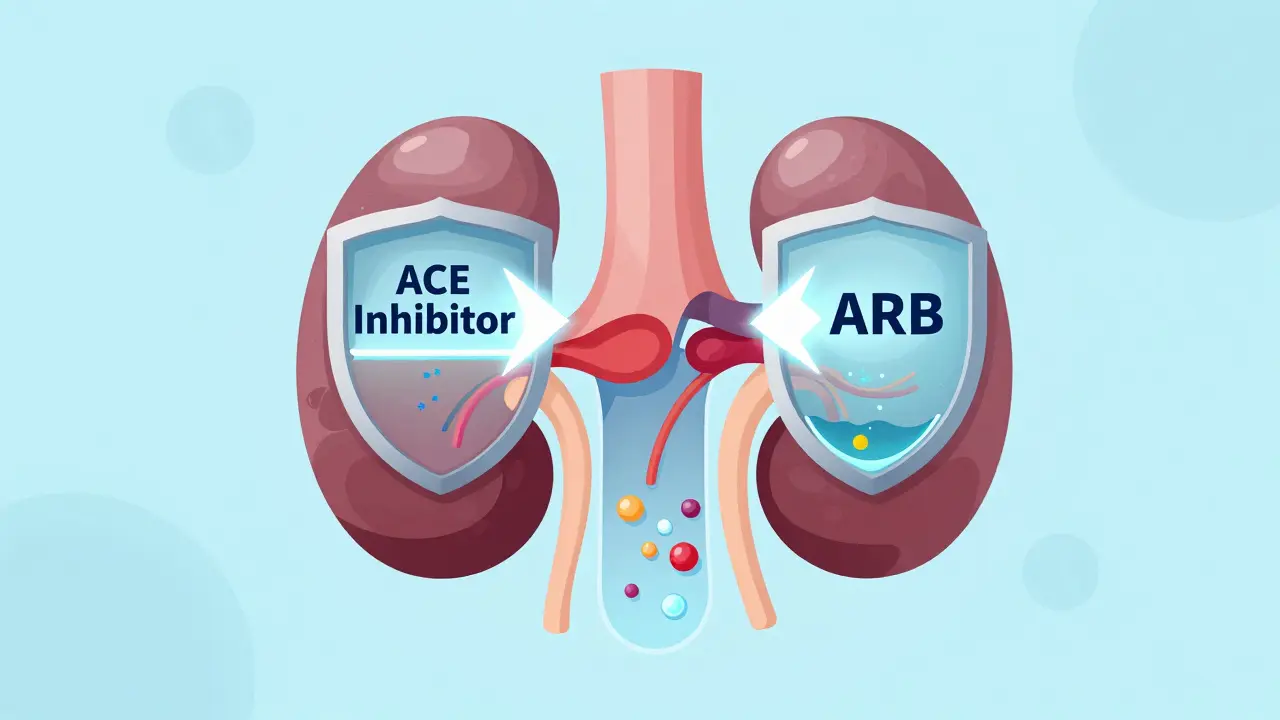
Diabetic Nephropathy: How ACE Inhibitors, ARBs, and Protein Control Protect Your Kidneys
ACE inhibitors and ARBs are proven to slow kidney damage in diabetes by reducing proteinuria and lowering internal kidney pressure. Used at maximum tolerated doses, they’re the cornerstone of diabetic nephropathy treatment-alongside careful protein control.