Carbamazepine Generics: Enzyme Induction and Drug Interactions Explained
 Jan, 3 2026
Jan, 3 2026
Switching from brand-name carbamazepine to a generic version might seem like a simple cost-saving move - but for many people, it’s anything but. Carbamazepine isn’t like other medications. Even small changes in how it’s made can throw off seizure control, trigger dangerous side effects, or cause interactions with other drugs you’re taking. The reason? Its powerful effect on liver enzymes and its narrow therapeutic window. If you or someone you know takes carbamazepine, understanding how generics work - and when they might not - could prevent hospital visits, breakthrough seizures, or worse.
Why Carbamazepine Is Different
Carbamazepine is used to treat epilepsy, trigeminal neuralgia, and some mood disorders. But unlike most drugs, it doesn’t just sit in your body and do its job. It actively changes how your liver works. Carbamazepine is a strong inducer of CYP3A4, one of the most important enzyme systems in your body. This enzyme breaks down about half of all prescription drugs - including blood thinners, birth control pills, antidepressants, and even some heart medications. When you start carbamazepine, your body begins making more of these enzymes within 48 to 72 hours. By the time you’ve been taking it for two to three weeks, your liver is working at full speed. That means other drugs you’re on get broken down faster - so they don’t work as well. For example, if you’re on warfarin for a blood clot, carbamazepine can drop its levels by up to 50%. That increases your risk of stroke. If you’re on birth control, you might get pregnant even if you take it perfectly. And here’s the kicker: carbamazepine also speeds up its own breakdown. This is called autoinduction. So your body starts needing more of the drug over time just to keep the same level in your blood. That’s why doctors often start you on a low dose and slowly increase it - not just to avoid side effects, but because your body is changing how it handles the drug.The Narrow Therapeutic Window
The safe and effective range for carbamazepine in your blood is incredibly tight: 4 to 12 micrograms per milliliter. Go below 4, and seizures can return. Go above 12, and you risk dizziness, double vision, nausea, or even life-threatening toxicity like bone marrow suppression or liver damage. That’s what makes carbamazepine a narrow therapeutic index (NTI) drug. Even a 10% change in blood levels can mean the difference between control and crisis. For comparison, most drugs have a much wider range - think of antibiotics or blood pressure pills. With carbamazepine, there’s no room for error. The problem? Generic versions of carbamazepine are allowed to differ in how quickly they release the drug into your system - as long as they fall within 80% to 125% of the brand-name version’s absorption. That sounds fine on paper. But when you’re dealing with a drug that has such a narrow window and complex metabolism, even small differences in absorption can push levels out of range. A 2018 study of 327 patients found that 12.4% had seizures or side effects after switching between different generic brands - even though all met FDA bioequivalence standards. Nearly 8% ended up in the emergency room. One patient’s level dropped from 7.2 to 4.8 mcg/mL after switching generics - same dose, same pharmacy, different manufacturer. That’s not a fluke. It’s a pattern.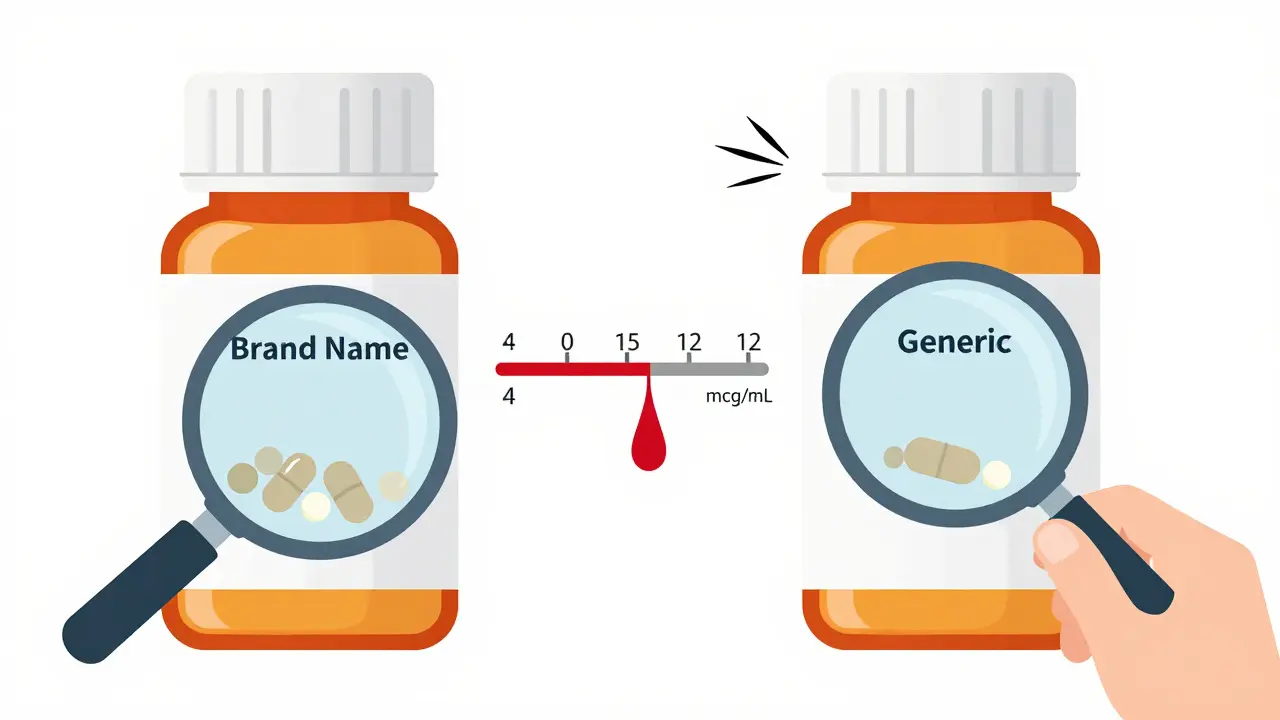
Generics Aren’t All the Same
There are over 30 FDA-approved generic versions of immediate-release carbamazepine and 18 of extended-release forms. Each one uses different fillers, binders, and coating technologies. Some are made as beads inside capsules; others are tablets with special coatings. These differences affect how the drug dissolves in your stomach - especially if you have gastroparesis, take antacids, or have other digestive issues. One patient on Reddit described how a generic made by Nostrum Pharmaceuticals had larger beads than others, which didn’t dissolve well for her because of slow stomach emptying. Her levels dropped, and her seizures came back. Her pharmacist didn’t know the difference - because most don’t track which specific generic they’re dispensing unless the doctor writes “dispense as written.” The European Medicines Agency (EMA) and many experts now treat carbamazepine differently than other generics. In Europe, steady-state bioequivalence studies are required - meaning they test the drug after weeks of use, not just a single dose. That’s more realistic because carbamazepine’s effects build over time. The U.S. FDA still mostly relies on single-dose studies in healthy volunteers - not people with epilepsy, liver disease, or on multiple other medications.Who’s Most at Risk?
Not everyone has problems switching generics. About 60% of patients do fine. But certain groups are far more vulnerable:- People with poorly controlled seizures - Even a small drop in carbamazepine levels can trigger a seizure cluster.
- Women of childbearing age - Hormones affect CYP3A4 activity. Studies show women have 22% higher rates of breakthrough seizures after switching generics.
- People taking other NTI drugs - If you’re also on lithium, valproic acid, or phenytoin, carbamazepine can mess with those levels too.
- Asian patients - If you’re of Han Chinese, Thai, Malaysian, or Filipino descent, you may carry the HLA-B*1502 gene variant. Taking carbamazepine with this gene increases your risk of Stevens-Johnson Syndrome - a life-threatening skin reaction - by 10 times. Screening is recommended before starting.
- Older adults and those with liver disease - Slower metabolism means carbamazepine builds up faster, increasing toxicity risk.
What You Can Do
If you’re on carbamazepine, here’s what actually works:- Ask your doctor to write “dispense as written” or DAW 1 on your prescription. This stops pharmacies from automatically substituting generics. Most neurologists in the U.S. do this for carbamazepine patients.
- Know your manufacturer. If your pharmacy switches to a different generic, call your doctor. Don’t assume it’s safe. Keep a note of the name on the pill bottle - Nostrum, Mylan, Teva, etc.
- Get your blood levels checked. Before switching, get a baseline level. Then check again 7 to 14 days after the switch. If your level changes by more than 15%, your dose may need adjusting. The International League Against Epilepsy recommends this.
- Track your symptoms. Keep a simple log: seizure frequency, dizziness, rash, nausea, fatigue. Even small changes matter.
- Don’t stop or change doses on your own. Autoinduction means your body adapts over time. Suddenly stopping can cause withdrawal seizures.
The Bigger Picture
Carbamazepine is one of the most prescribed antiepileptic drugs in the U.S., but 92% of prescriptions are for generics. The cost is low - about $8.50 for a 60-day supply of 200 mg tablets. But the hidden costs? Emergency visits, hospitalizations, lost work, and preventable seizures. The FDA now lists carbamazepine extended-release products as “high-priority” for better testing methods. Researchers are developing genetic tests to predict how fast someone metabolizes the drug. One study found people with a specific CYP3A4 gene variant need 25% less drug to reach safe levels. In the next five years, precision dosing - using your genes, weight, age, and other medications - will likely become standard. Until then, the safest approach is simple: stay on the same brand and manufacturer. If you must switch, monitor closely. Your life depends on it.Can I switch between different generic brands of carbamazepine safely?
It’s risky. Even though generics meet FDA bioequivalence standards, carbamazepine has a narrow therapeutic window and autoinduces its own metabolism. Studies show 12-15% of patients experience breakthrough seizures or side effects after switching between generic manufacturers. Always consult your doctor before switching and get a blood level check 7-14 days after the change.
Why does carbamazepine interact with so many other drugs?
Carbamazepine strongly induces CYP3A4 and other liver enzymes that break down drugs. This includes blood thinners like warfarin, antidepressants, birth control pills, statins, and immunosuppressants. As a result, these drugs may become less effective. Always tell your doctor and pharmacist about every medication you take - including over-the-counter pills and supplements.
How long does it take for carbamazepine to start affecting other drugs?
Enzyme induction begins within 48-72 hours of starting carbamazepine, but it takes 2-3 weeks to reach full effect. This means interactions may not show up right away. If you start carbamazepine while on another drug, your doctor may need to increase that drug’s dose over time - or switch to a safer alternative.
Should I get tested for the HLA-B*1502 gene before taking carbamazepine?
If you’re of Asian descent - especially Han Chinese, Thai, Filipino, Malaysian, or Indian - yes. Carrying the HLA-B*1502 gene increases your risk of Stevens-Johnson Syndrome by 10 times. The FDA recommends screening before starting carbamazepine in these populations. If positive, your doctor should choose a different medication like levetiracetam or lamotrigine.
Is therapeutic drug monitoring really necessary for carbamazepine?
Yes - especially if you’re switching generics, changing doses, or adding/removing other medications. Carbamazepine levels vary widely between people, even on the same dose. Monitoring every 7-14 days after a change helps prevent under- or overdosing. The therapeutic range is 4-12 mcg/mL, but optimal levels vary per person. Don’t rely on how you feel alone.
What should I do if I notice my seizures getting worse after switching generics?
Don’t wait. Contact your neurologist immediately. Your carbamazepine level may have dropped. Bring your pill bottle - the manufacturer name matters. You may need a blood test and a dose adjustment. In many cases, going back to your original generic or brand-name version restores control. Keep a seizure diary to help your doctor track patterns.
Peyton Feuer
January 4, 2026 AT 06:27man i switched generics last year and my seizures got worse for like 3 weeks. i thought i was losing it until my neurologist checked my levels and they dropped 20%. now i always check the bottle before i leave the pharmacy. dont trust the system.
Shanna Sung
January 5, 2026 AT 16:57the FDA is in bed with big pharma. they dont want you to know generics can kill you. they test on healthy college kids who dont even take meds. my cousin died after switching. they called it 'natural progression'. lol
John Ross
January 5, 2026 AT 23:06Carbamazepine’s CYP3A4 autoinduction profile is a pharmacokinetic nightmare-especially when combined with NTI comorbidities. The 80–125% bioequivalence window is statistically valid for most drugs, but for a compound with nonlinear metabolism and narrow TI, it’s clinically indefensible. EMA’s steady-state requirement is the gold standard; the FDA’s single-dose paradigm is archaic.
Ashley Viñas
January 5, 2026 AT 23:33if you're taking carbamazepine and you didn't get genetic tested for HLA-B*1502, you're basically playing russian roulette with your skin. i know people who thought 'it won't happen to me'-and then they ended up in the burn unit. just get the test. it's $150. your face is worth more.
Mandy Kowitz
January 7, 2026 AT 01:30so you're telling me i have to pay extra to not die? thanks capitalism. next you'll tell me i need to buy a special spoon to eat my meds.
Justin Lowans
January 8, 2026 AT 16:52This is one of the most important public health discussions we’ve had in neurology in decades. The disparity between regulatory standards and clinical reality is staggering. Thank you for laying this out with such clarity. It’s not just about seizures-it’s about dignity, autonomy, and trust in the medical system.
Michael Rudge
January 10, 2026 AT 11:07you people are hysterical. if you can’t handle a generic, maybe you shouldn’t be on a drug that requires a PhD to manage. just take the brand. problem solved. oh wait-you’re too cheap for that, huh?
Ethan Purser
January 12, 2026 AT 08:33we’re all just atoms in a system that doesn’t care if we live or die. carbamazepine isn’t the problem-it’s the illusion of control. the pill bottle says ‘equivalent’ but your body knows the truth. you’re not a patient-you’re a data point. and the system is always optimizing for profit, not people.
Terri Gladden
January 14, 2026 AT 03:48i switched to a new generic and my face broke out in this weird rash and i thought it was just stress but then i read this and i freaked out and called my doc and they said ohhh that’s the HLA thing you shoulda been tested for. i feel like a moron. please get tested. please.
Jennifer Glass
January 14, 2026 AT 19:10it’s wild how a drug that’s been around since the 60s still has so many unanswered questions. we treat it like a black box, but it’s really a living system inside us-changing, adapting, reacting. maybe the real issue isn’t the generic-it’s that we’ve stopped listening to the body’s signals.
melissa cucic
January 16, 2026 AT 19:07It is imperative, from both a clinical pharmacology and ethical standpoint, that regulatory agencies revise their bioequivalence criteria for narrow-therapeutic-index drugs-particularly those exhibiting autoinduction kinetics. The current paradigm, rooted in 1980s pharmacokinetic models, is demonstrably inadequate for modern polypharmacy environments.
Akshaya Gandra _ Student - EastCaryMS
January 18, 2026 AT 04:10im from india and we use a lot of generics here. my aunt had seizures after switch. now we only use one brand. i didnt know this was a thing in usa too. thanks for sharing
en Max
January 19, 2026 AT 12:49As a pharmacist with over 18 years of experience in neuropharmacotherapy, I can confirm: the data is unequivocal. Generic substitution for carbamazepine-particularly across multiple manufacturers-represents a measurable and preventable risk factor for therapeutic failure. I routinely counsel patients to retain the same manufacturer, and I document the specific lot and manufacturer in the electronic record. This is not paranoia-it is precision medicine.