Why Medications Affect People Differently: The Real Science Behind Drug Side Effects
 Dec, 15 2025
Dec, 15 2025
Medication Metabolism Risk Calculator
This calculator estimates your risk of side effects based on how your body processes medications. It's designed to help you understand the science behind why drugs affect people differently. It is not a substitute for medical advice or genetic testing.
Your Risk Assessment
How this works
Your body's ability to process medications depends on genes that control enzymes like CYP2D6 and CYP2C9. Poor metabolizers build up drugs faster, increasing side effect risks. Ultra-rapid metabolizers clear drugs too quickly, reducing effectiveness.
Have you ever taken the same medication as someone else and had a completely different experience? One person feels fine, another ends up in the hospital. It’s not just bad luck. It’s biology. The reason some people get sick from a drug while others don’t has everything to do with how their body is built - down to their genes.
It’s Not Just the Drug - It’s You
Medications don’t work the same way for everyone. That’s not a myth. It’s science. A drug meant to lower blood pressure might make one person dizzy, while another feels nothing at all. An antibiotic could cause a rash in one person and work perfectly in another. These aren’t rare oddities. They’re the norm.The World Health Organization defines an adverse drug reaction as any harmful, unintended response to a medicine taken at normal doses. In the U.S., these reactions are the fourth leading cause of death. In Europe, 3.6% of hospital admissions are directly linked to them. And in Australia, the cost of managing these reactions runs into hundreds of millions annually. The problem isn’t that drugs are unsafe - it’s that we treat them like they’re one-size-fits-all.
Your Genes Are the Key
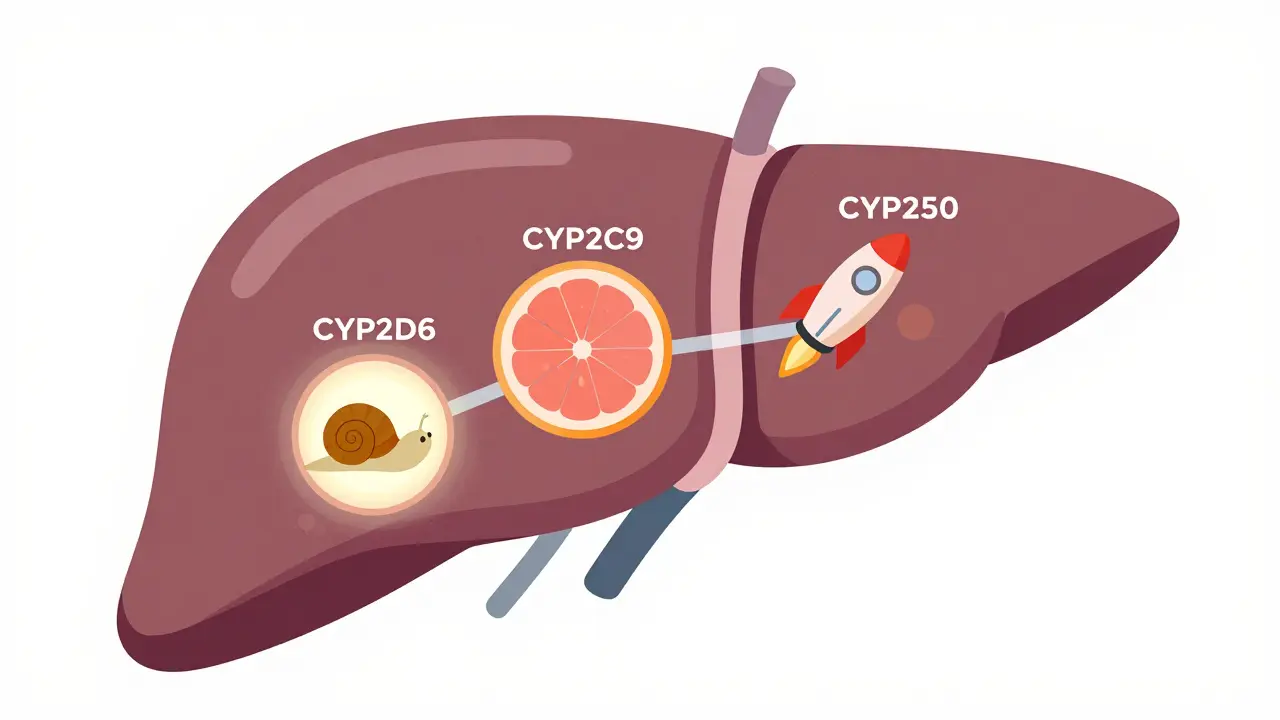
Your DNA holds the blueprint for how your body handles drugs. The most important players are enzymes in your liver, especially the cytochrome P450 family - CYP2D6, CYP2C9, and CYP2C19. These enzymes break down over 70% of all prescription medications. But not everyone has the same versions of these enzymes.Some people are poor metabolizers. Their bodies break down drugs too slowly. That means the drug builds up, increasing the risk of side effects. About 5 to 10% of Caucasians are poor metabolizers of CYP2D6. If they take a common antidepressant like amitriptyline, they can end up with toxic levels in their blood - even on a normal dose.
Others are ultra-rapid metabolizers. Their bodies clear drugs too fast. For them, standard doses might not work at all. In some populations, like Ethiopians, up to 29% are ultra-rapid metabolizers of CYP2D6. A standard dose of codeine? It turns into morphine too quickly, causing dangerous breathing problems in children - a tragedy that led to FDA warnings in 2013.
And then there’s warfarin, the blood thinner. Two people on the same dose can have wildly different outcomes. One stays stable, the other bleeds internally. Why? Because of two genes: CYP2C9 and VKORC1. Together, they explain 30 to 50% of why warfarin doses vary so much. When doctors test for these variants, patients reach safe levels faster - and have 31% fewer major bleeds.
It’s Not Just Genes - Age, Diet, and Other Drugs Matter Too
Your genes aren’t the whole story. Your age, what you eat, and other medications you take all change how drugs behave in your body.As you get older, your body changes. Fat increases. Liver and kidney function slow down. That means fat-soluble drugs like diazepam or antidepressants stick around longer. An 80-year-old might need half the dose of a 30-year-old for the same effect.
Then there’s diet. Grapefruit juice? It blocks an enzyme called CYP3A4. That can cause statins like simvastatin to build up to dangerous levels. One glass can turn a safe dose into a toxic one.
And drug interactions? They’re a silent killer. Take amiodarone - a heart rhythm drug - and warfarin together. Amiodarone shuts down the enzyme that clears warfarin. That can boost warfarin levels by 100 to 300%. The result? A spike in INR, internal bleeding, emergency rooms.
Even inflammation from a cold or flu can lower enzyme activity by 20 to 50%. So a drug that worked fine last week might suddenly cause side effects because your body is fighting an infection.
Pharmacogenomics: The Future Is Here - But Not Everywhere
This is where pharmacogenomics comes in. It’s the science of using your genes to choose the right drug and dose. The FDA now includes pharmacogenomic info on the labels of over 300 drugs. For 44 of them, they give specific dosing recommendations based on genetics.At St. Jude Children’s Research Hospital, testing for a gene called TPMT before giving mercaptopurine to kids with leukemia cut severe side effects from 25% to just 12%. That’s life-changing.
And it’s not just cancer. In psychiatry, testing for CYP2D6 and CYP2C19 helps doctors pick antidepressants that won’t make patients worse. In cardiology, testing for CYP2C19 before giving clopidogrel (a common heart drug) identifies patients who won’t benefit from it at all - about 2 to 15% of people. These patients need a different drug, not a higher dose.
But here’s the problem: most doctors still don’t test. Only 18% of U.S. insurers cover it. Two-thirds of physicians say they don’t feel trained to use genetic results. And hospitals? Only 32% have systems that alert doctors when a patient’s genetics suggest a dangerous interaction.
It’s not that the science is missing. It’s that the system hasn’t caught up.
Why Some People Waste Money on Drugs That Don’t Work
This isn’t just about safety. It’s about money.Take zafirlukast, a drug for asthma. It costs $250 to $300 a month. But only about 5% of asthma patients have a specific gene variant - the 5-LO polymorphism - that makes it work. The rest? They pay hundreds a month for no benefit. That’s billions wasted every year.
Same with leukotriene modifiers. A 2022 Mayo Clinic study found that patients who got genetic testing had 32% fewer ER visits and 26% shorter hospital stays. But without testing, doctors are guessing. And guessing costs lives - and money.

What’s Changing - And What’s Still Broken
The good news? Things are moving. In January 2024, Medicare started covering pharmacogenomic testing for 17 high-risk drugs. The cost of genetic panels has dropped from $2,000 in 2015 to under $250 today. The FDA approved the first point-of-care CYP2C19 test in 2023 - results in 60 minutes. The EU now requires pharmacogenomic data in all new clinical trials.But the biggest barrier isn’t science. It’s access. Genetic testing is still mostly available in big hospitals and academic centers. Rural clinics, community pharmacies, and low-income patients? They’re left out. And even when testing is done, results often sit in a file, unread by the doctor who prescribed the drug.
And here’s the hard truth: genes aren’t the whole picture. Most drug responses involve hundreds of small genetic changes, not just one. Single-gene tests explain maybe 15% of side effects. The rest? We’re still figuring it out.
What You Can Do Right Now
You don’t need a genetics degree to protect yourself.- If you’ve had a bad reaction to a drug, tell your doctor - and write it down. Include the drug, the side effect, and when it happened.
- Ask if your meds are on the FDA’s pharmacogenomic list. You can search it online - it’s public.
- If you’re on multiple drugs (especially if you’re over 65), ask about possible interactions. Many side effects aren’t from one drug - they’re from the mix.
- If you’re considering a new drug, ask: “Is there a genetic test that could help decide if this is right for me?”
- Keep a list of all your meds - including supplements and over-the-counter drugs. Bring it to every appointment.
There’s no magic pill. But knowing your body’s unique response to drugs is the closest thing.
Why do some people have side effects from drugs while others don’t?
It comes down to genetics, age, other medications, diet, and even infections. Your liver enzymes - shaped by your DNA - determine how fast or slow your body breaks down a drug. Some people process drugs too slowly, leading to buildup and toxicity. Others process them too fast, making the drug ineffective. Environmental factors like grapefruit juice or inflammation can also change how drugs work.
Can genetic testing prevent bad drug reactions?
Yes, in specific cases. For drugs like warfarin, clopidogrel, and certain antidepressants, genetic testing can reduce serious side effects by up to 30%. For example, testing for CYP2C9 and VKORC1 before starting warfarin cuts dangerous bleeding events by 31%. In pediatric cancer, testing for TPMT before giving mercaptopurine cut severe toxicity from 25% to 12%.
Are pharmacogenomic tests covered by insurance?
Coverage is improving but still limited. As of 2024, Medicare covers pharmacogenomic testing for 17 high-risk medications. Private insurers cover it in only about 18% of cases. Most tests cost under $250 now, but without insurance, you may pay out-of-pocket. Check with your provider and ask if your drug is on the FDA’s pharmacogenomic list - that increases the chance of coverage.
Which drugs have genetic testing recommendations?
The FDA has labeled over 300 drugs with pharmacogenomic information, and 44 have specific dosing guidelines based on genetics. Key examples include warfarin (CYP2C9/VKORC1), clopidogrel (CYP2C19), amitriptyline and SSRIs (CYP2D6), codeine (CYP2D6), and mercaptopurine (TPMT). Always ask your pharmacist or doctor if your prescription is one of them.
Should I get genetic testing before taking any new medication?
Not always - but it’s worth asking if you’ve had bad reactions before, are on multiple medications, or are taking a high-risk drug like warfarin, clopidogrel, or certain antidepressants. Testing is most helpful when it changes your treatment plan. For routine antibiotics or pain relievers, it’s usually not needed. Talk to your doctor about your personal risk factors and whether testing makes sense for you.
Can I get tested without a doctor’s order?
Some direct-to-consumer companies offer pharmacogenomic panels, but results without medical interpretation can be misleading. A gene variant might suggest a risk, but only a doctor or pharmacist can tell you what it means for your specific meds. Always bring test results to your healthcare provider - don’t change your dose based on a kit alone.
Is pharmacogenomics only for older adults?
No. While older adults are at higher risk due to multiple medications and slower metabolism, pharmacogenomics matters at any age. Children with cancer, teens on antidepressants, and young adults on birth control or blood thinners can all benefit. In fact, testing in pediatric oncology has already saved lives by preventing deadly toxicities.
Benjamin Glover
December 16, 2025 AT 11:42This is why Britain’s NHS should never adopt this genetic nonsense. We’ve been giving the same doses for decades and people are fine. Stop overcomplicating medicine with American gene-hype.
My grandad took warfarin for 20 years without a single test. He’s still alive. Your ‘science’ is just expensive guesswork dressed up as innovation.
Sai Nguyen
December 17, 2025 AT 03:08India doesn’t need this. We don’t have the infrastructure to test everyone. We fix problems with common sense, not DNA reports.
My cousin took amitriptyline and got dizzy? He stopped taking it. Done. No test needed. You Americans turn every pill into a genetic mystery.
Lisa Davies
December 17, 2025 AT 17:45This is SO important!! 🙌 I had a nightmare with SSRIs until my doctor ran a CYP2D6 test - turned out I’m a poor metabolizer. Switched meds and my life changed. 💙
Why isn’t this standard everywhere?? Everyone deserves to know how their body will react. This isn’t sci-fi - it’s basic care. 🧬❤️
Jake Sinatra
December 19, 2025 AT 14:32The data presented here is methodologically sound and aligns with peer-reviewed literature from the Journal of Clinical Pharmacology and the New England Journal of Medicine. The integration of pharmacogenomics into clinical practice represents a paradigm shift in personalized medicine.
However, the implementation challenges cited - including physician training gaps and insurance coverage limitations - remain significant systemic barriers. Policy reform must precede widespread adoption.
Further longitudinal studies are warranted to quantify long-term cost-benefit outcomes across diverse populations.
RONALD Randolph
December 20, 2025 AT 13:06Stop. Just stop. This isn’t ‘science.’ It’s corporate greed wrapped in a lab coat. The FDA doesn’t ‘recommend’ anything - they’re pressured by Big Pharma to sell more tests.
And who pays for it? YOU. The patient. The same people who can’t afford insulin. This is a scam. One gene, one test, one profit margin. Wake up.
Also - grapefruit juice? That’s been known since the 1990s. You’re acting like this is new. It’s not. It’s just marketing.
Raj Kumar
December 20, 2025 AT 19:06Bro, I work in a rural clinic in Uttar Pradesh. We don’t have genetic testers, but we do have patients who tell us what happened last time they took a pill.
So we listen. We start low. We watch. We adjust. That’s pharmacogenomics without the fancy machine.
And honestly? Most docs here know this already. We just don’t call it ‘pharmacogenomics’ because we’re too busy keeping people alive.
Christina Bischof
December 22, 2025 AT 02:32Jocelyn Lachapelle
December 22, 2025 AT 10:04Thank you for writing this. I’ve been on 5 different antidepressants and each one either did nothing or made me feel like a zombie.
Finally got tested last year - turns out I’m a CYP2C19 poor metabolizer. Switched to one that doesn’t rely on that enzyme and I’m actually sleeping again.
It’s not magic. It’s just knowing your body a little better. And that’s worth so much.
Mike Nordby
December 23, 2025 AT 07:06The assertion that pharmacogenomic testing reduces adverse events by 30% is supported by multiple randomized controlled trials, including the IGNITE Network studies and the Mayo Clinic’s PREDICT program. However, the claim that single-gene variants explain only 15% of variability is accurate and underscores the complexity of polygenic drug response.
Current clinical guidelines from CPIC and DPWG recommend testing for specific drug-gene pairs, but broader implementation requires EHR integration and clinician decision support tools - not just access to testing.
Furthermore, environmental modifiers - such as inflammation-induced CYP suppression - remain underutilized in risk stratification models. Future iterations must incorporate dynamic biomarkers alongside static genotypes.